S p d f orbitals shapes 177931-Describe the shapes of s p d and f orbitals
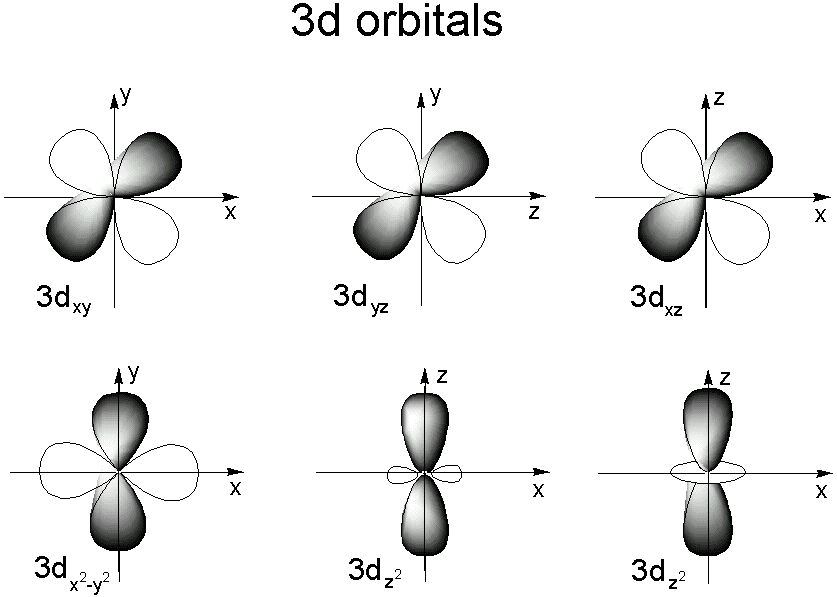
The type (or shape) of orbital the electron resides in Each value for the angular momentum quantum number will correspond specifically with s,p,d, or f orbitals, which each have their own distinct shapesFor example, 3d xy, 3d yz, 3d zx, 3d x 2y 2 and 3d z 2All levels except the first have p orbitals d ORBITALS In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3px, 3py, 3pz)
Shapes Of Orbitals And Sublevels
Describe the shapes of s p d and f orbitals
Describe the shapes of s p d and f orbitals-For a p orbital, draw a figure eight;The type of sublvel (s,p,d,f) orbitals with different shapes occupy different regions these regions are called _____ sublevels the quantum numbers designated in ascending order use the letters _____ s,p,d,f what is the shape of the s orbital?
:max_bytes(150000):strip_icc()/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)


S P D F Orbitals And Angular Momentum Quantum Numbers
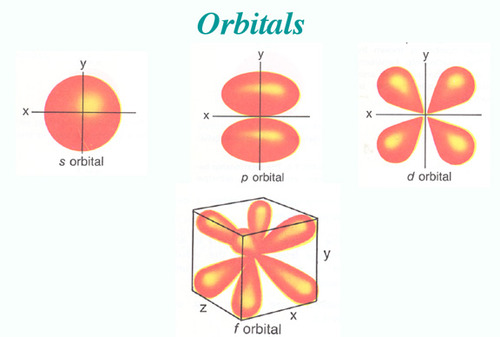
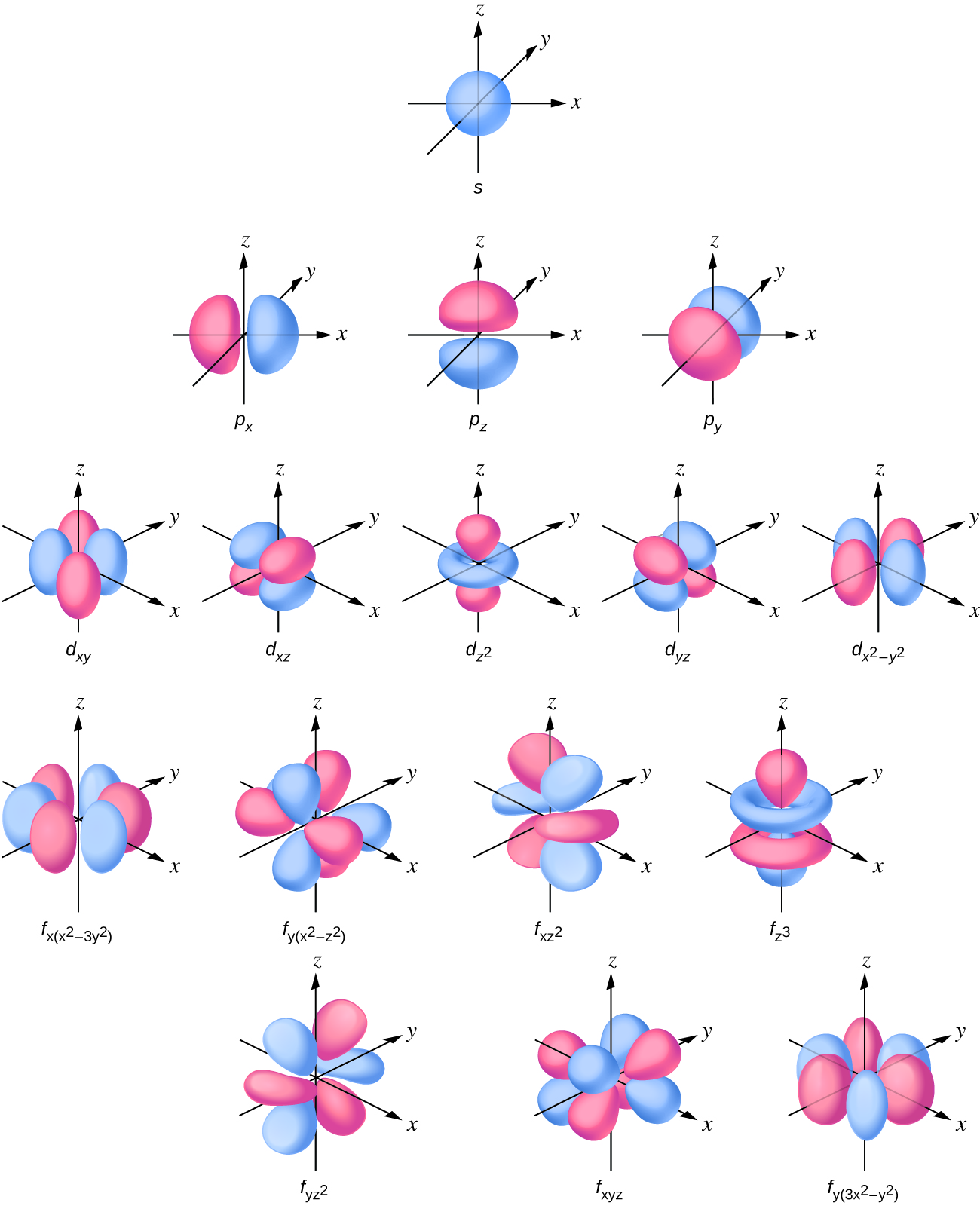
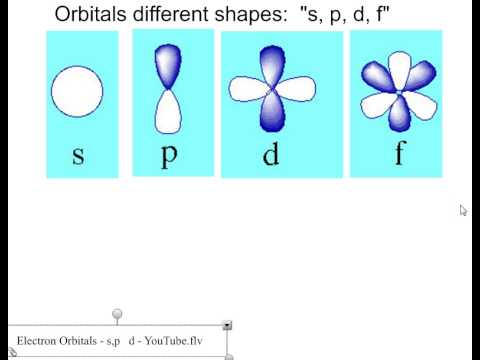
List the four orbital shapes The orbital shapes are s, p, d, and f Summarize Aufbau's rule for filling orbitals Electrons fill orbitals with the lowest energy level possible firstLarger elements have additional orbitals, making up the third electron shell Subshells d and f have more complex shapes and contain five and seven orbitals, respectively Principal shell 3n has s, p, and d subshells and can hold 18 electrons Principal shell 4n has s, p, d, and f orbitals and can hold 32 electronsFor an f orbital, see below An s orbital is a sphere In two dimensions, we draw it as a circle A p orbital consists of two lobes of electron density on either side of the nucleus We usually draw p orbitals as figure eights, but we should remember p orbitals are really much
Relative probability is indicated by a series of dots, indicating the "electron cloud" 90% electron probability/cloud for 1s orbital (notice higher probability toward the centre) p orbitals and d orbitals p orbitals look like a dumbell with 3 orientations px, py, pz ("p sub z") Four of the d orbitals resemble two dumbells in a cloverS, p, d, f and so on are the names given to the orbitals that hold the electrons in atoms These orbitals have different shapes (eg electron density distributions in space) and energies (eg 1s is lower energy than 2s which is lower energy than 3s;For example, 3d xy, 3d yz, 3d zx, 3d x 2y 2
S, p, d, f and so on are the names given to the orbitals that hold the electrons in atoms These orbitals have different shapes (eg electron density distributions in space) and energies (eg 1s is lower energy than 2s which is lower energy than 3s;Maximum 6 electrons in 3 orbitals Maximum 2 electrons in 1 orbital Maximum 10 electrons in 5 orbitalsFred Senese of Antoine Frostburg explains "You might expect that the 's' stands for 'spherical' and 'p' stands for 'polar' because these imply the shapes of the s and p orbitals, but unfortunately, the letter designations have nothing to do with
:max_bytes(150000):strip_icc()/4fz3-electron-orbital-117451436-587f69f23df78c17b6354ebd-f7499851032246f5bbe03f1ffba963d5.jpg)


S P D F Orbitals And Angular Momentum Quantum Numbers


Organic Chemistry Atomic Structure Atoms And Atomic Orbitals Sparknotes
Protons forming in nucleus The f orbital has 15 protons to complete a fifth level of a tetrahedral structure Shape The f orbital is more complex, but follows the same rules based on proton alignment as the p and d orbitals When completely full it is similar to the d orbital, but cut in half (eight lobes instead of four)These letters are s, p, d, f, g, h, I, j, and many more but here, we are only looking at letters p, s, and d and their corresponding shapes You should know that it is impossible to draw an orbital because an electron is capable of taking up all space but we can draw the shape that it takes up most of the time, presumably 90% of the timeThe shapes of the other orbitals are more complicated The letters s, p, d, f, originally were used to classify spectra descriptively into series called sharp, principal, diffuse, and fundamental, before the relation between spectra and atomic electron configuration was known



The Actinide Research Quarterly 1st Quarter 04


Molecular Structure Atomic Orbitals
3D model to visualise the shapes of atomic orbitals s, p and dThe letters s, p, d, and f were assigned for historical reasons that need not concern us All we have to do is remember the shapes that correspond to each letter Since an electron can theoretically occupy all space, it is impossible to draw an orbital All we can do is draw a shape that will include the electron most of the time, say 95% of the time We call this shape the 95% contour s ORBITALSDescribe the shapes and relative energies of the s, p, d, and f atomic orbitals vscogirl vscogirl 12//19 Chemistry Middle School 47 Describe the shapes and relative energies of the s, p, d, and f atomic orbitals 1 See answer vscogirl is waiting for your help Add your answer and earn points oyinade oyinade



Ppt S P D F Orbitals Powerpoint Presentation Free Download Id



Parsing The Spdf Electron Orbital Model Chemistry Education Chemistry Classroom Teaching Chemistry
2s is lower energy than 2p)(image source)So for example,These letters are s, p, d, f, g, h, I, j, and many more but here, we are only looking at letters p, s, and d and their corresponding shapes You should know that it is impossible to draw an orbital because an electron is capable of taking up all space but we can draw the shape that it takes up most of the time, presumably 90% of the timeShapes of Orbitals and Electron Density Patterns The s orbitals are spherical, while p orbitals are polar and oriented in particular directions (x, y, and z) It may be simpler to think of these two letters in terms of orbital shapes (d and f aren't described as readily)However, if you look at a crosssection of an orbital, it isn't uniform


Bohr Model S P D F Orbitals Flashcards Quizlet


Untitled Document
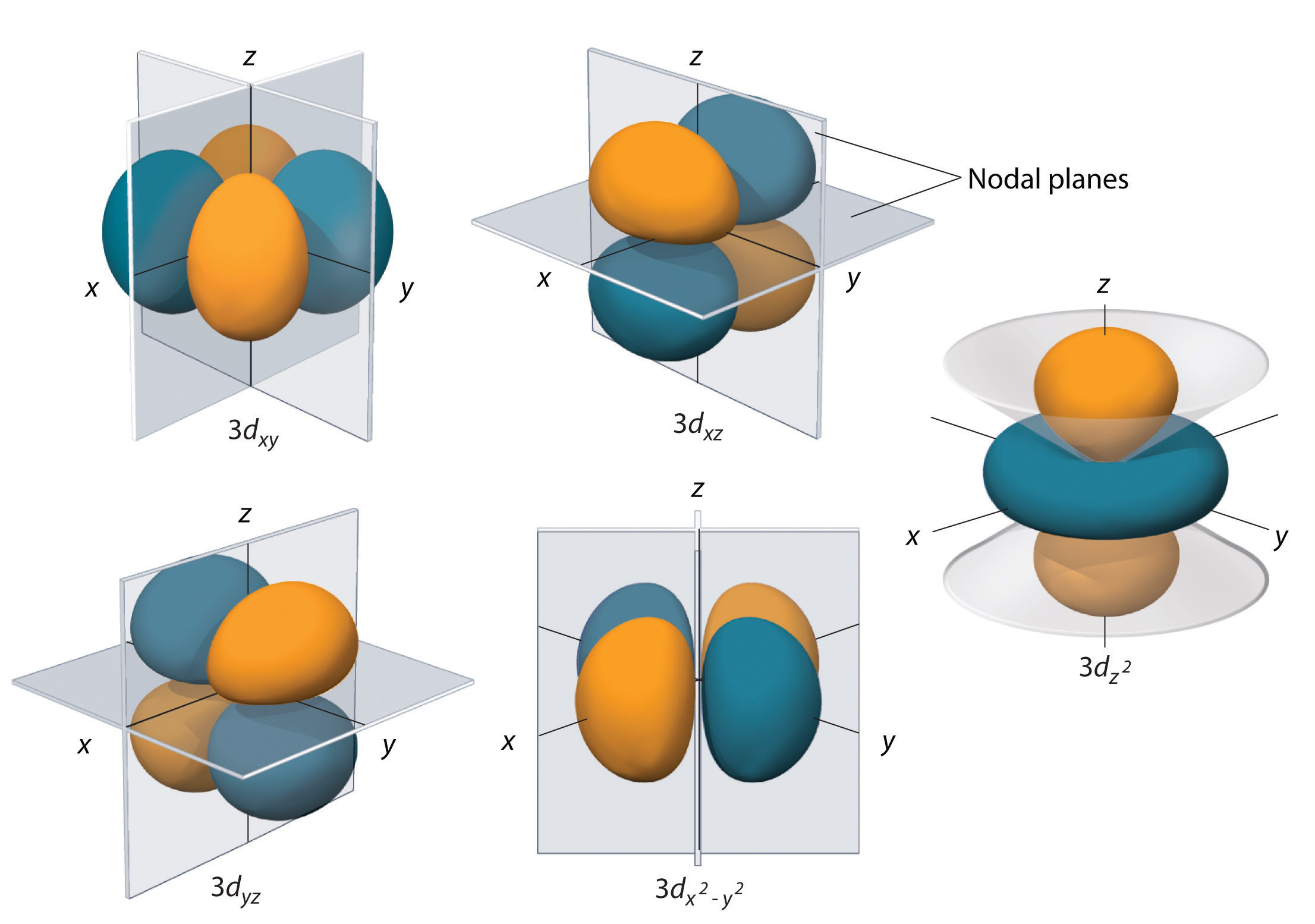
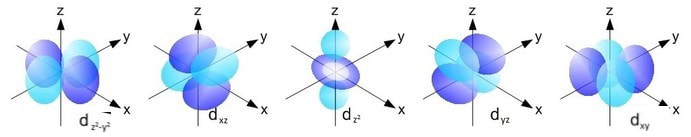
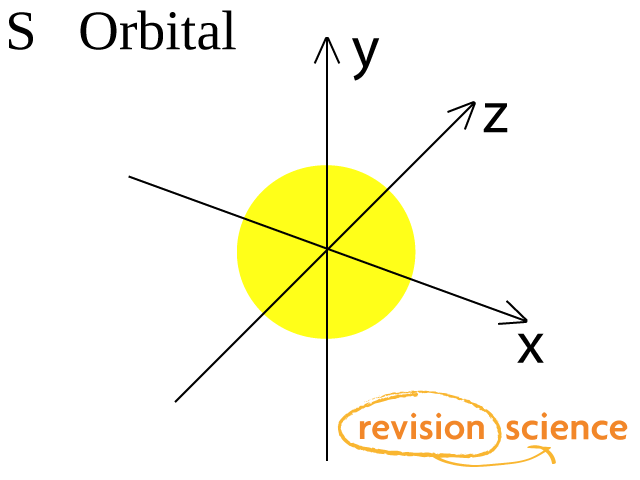
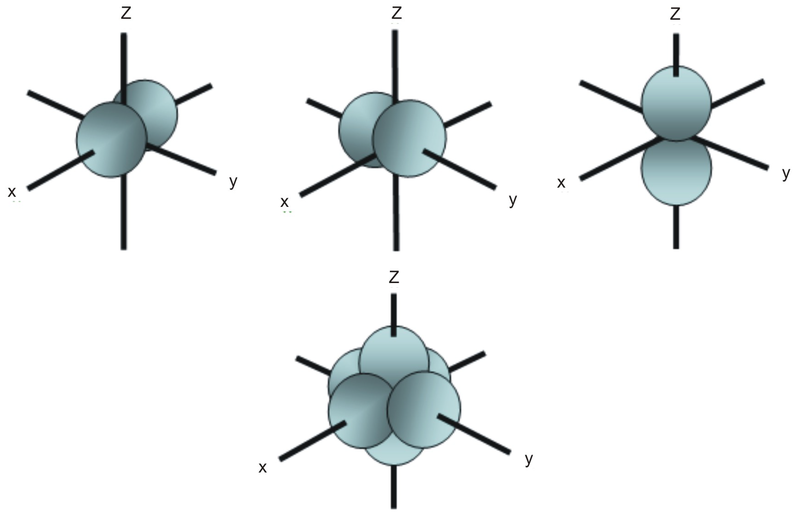
The sorbitals are solid spherical shape around the nucleus When principal quantum number n = 1 and azimuthal quantum number l = 0, that is 1s orbital which is closest to the nucleus When n = 2 and l = 0 , ie 2s orbital which contains one node When n = 3 and l = 0, ie 3s orbital which contains two nodesThese are designated as p orbitals and have dumbbell shapes Each of the p orbitals has a different orientation in threedimensional space d Orbitals When l = 2, m 1 values can be −2, −1, 0, 1, 2 for a total of five d orbitals Note that all five of the orbitals have specific threedimensional orientations f OrbitalsThe porbitals of higher energy levels have similar shapes although their size are bigger Shape of dorbitals For dsubshell, l = 2, there are five values of m namely 2, 1, 0, 1, 2 It means d orbitals can have five orientations These are represented by d xy, d yz, d zx, d x 2y 2 and d z 2;
:max_bytes(150000):strip_icc()/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)


S P D F Orbitals And Angular Momentum Quantum Numbers


Parsing The Spdf Electron Orbital Model
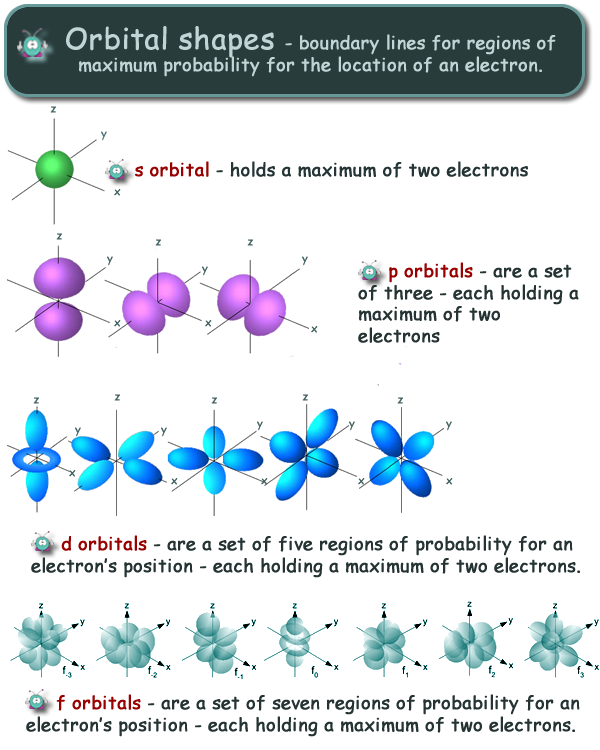
Shapes Of S P And D Orbitals Pdf Download >>> DOWNLOAD (Mirror #1) d9ef92e1f7 embarkation card japan pdf downloadlouis claude fillion pdf downloadthe soul's code pdf downloadtoeic 4n4 860 level pdf downloadthe columbian exchange and global trade pdf downloadmcconnell economics 19th edition pdf free downloadghostgirl christmas spirit pdf downloadmanierismo y barroco pdf downloadcefaleaQuestion Question 3 A) Sketch The Shapes Of The S, P And D Orbitals State How Many Electrons Each Individual Orbital Can Contain As Well The Total Number In A Full S, P And D Subshell 10 Marks B) From The Following Section Of The Periodic Table He Be B с F Ne Sono 3 1 AI Si CI Show How The P Electrons Are Arranged In Be, B, C, N, O, F, Ne And State TheThe subshells s, p, d, and f contain the following number of orbitals respectively, where every orbital can hold up to two electrons maximum s 1 orbital, 2 electrons p 3 orbitals, 6 electrons d 5 orbitals, 10 electrons f 7 orbitals, 14 electrons


7 6 The Shape Of Atomic Orbitals Chemistry Libretexts



You Are At Miracle Mbalisi S Blog Now Feel Free Understanding Atomic Orbitals Spdf And Their Shape
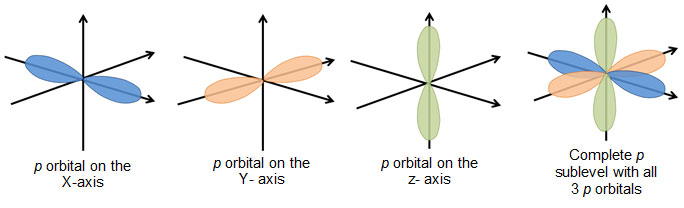
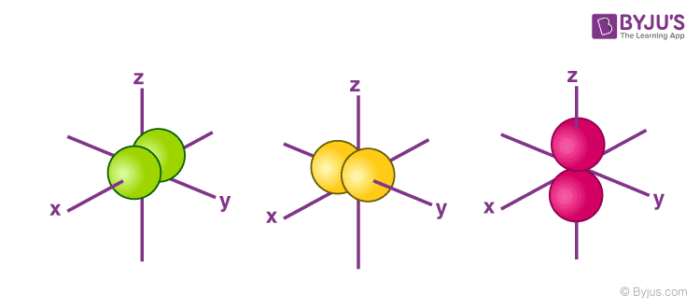
For p orbital Azimuthal quantum number l = 1 and the magnetic quantum number m = 1, 0, 1 Hence p orbitals have three orientations in space Thus p orbital corresponds to dumbbelled shape with the atomic nucleus at its center p orbitals have two lobes directed on opposite sides of the nucleusHere you will learn all about your basic ideas, techniques, termiNow consider sin 2 x, the square of the original functionIn quantum chemistry Ψ 2 provides us with the electron density it defines the size and shapes of the familiar orbitals s, p, d, f, etc


Shapes Of Orbitals S P D Shapes


Difference Between Atomic Orbital And Molecular Orbital Definition Characteristics Properties
An illustration of the shape of the 3d orbitals Click the images to see the various 3d orbitals There are a total of five d orbitals and each orbital can hold two electrons The transition metal series is defined by the progressive filling of the 3d orbitalsThese five orbitals have the following m l values m l =0, ±1, ±2,The subshells have four types s, p, d, and f, and each subshell has a specific number of orbitals with different shapes This is determined by the magnetic quantum number This is determined byThere are four different kinds of orbitals, denoted s, p, d and f each with a different shape Of the four, s and p orbitals are considered because these orbitals are the most common in organic and biological chemistry An sorbital is spherical with the nucleus at its centre, a porbitals is dumbbellshaped and four of the five d orbitals are cloverleaf shaped



What Is Spdf Configuration Chemistry Stack Exchange


Science Skool Atomic Orbitals
2s is lower energy than 2p)(image source)So for example,And if l is the state, then amsa bell is the city, and I am so pest would be what street you what street it lives on So we know that, um or in depth and orbital is the size Okay, so we can have really we really big or bills So there's the three shapes of the s orbital So as is a sphere Okay, the P or bill is kind of a dumb bell looking thingThe porbitals of higher energy levels have similar shapes although their size are bigger Shape of dorbitals For dsubshell, l = 2, there are five values of m namely 2, 1, 0, 1, 2 It means d orbitals can have five orientations These are represented by d xy, d yz, d zx, d x 2y 2 and d z 2;



12 1 5 Draw The Shape Of An S Orbital And The Shapes Of The P X P Y And P Z Orbitals Youtube


Atomic Structure Atoms And Atomic Orbitals Sparknotes
The psublevel is made up of a 3 identical dumbbell like orbitals Each one is situated on its own axis They are at 90 o angles from one and other The dsublevel is made up of a 5 different orbitals and the sublevel holds a maximum of 10 electrons The fsublevel is made up of a 7 different orbitals and holds a maximum of 14 electronsS Orbital Versus P Orbital While orbital numbers (eg, n = 1, 2, 3) indicate the energy level of an electron, the letters (s, p, d, f) describe the orbital shape The s orbital is a sphere around the atomic nucleus Within the sphere there are shells in which an electron is more likely to be found at any given time The smallest sphere is 1sAn illustration of the shape of the 3d orbitals Click the images to see the various 3d orbitals There are a total of five d orbitals and each orbital can hold two electrons The transition metal series is defined by the progressive filling of the 3d orbitalsThese five orbitals have the following m l values m l =0, ±1, ±2,



Shape Of The P1 2 Orbital Chemistry Stack Exchange


How Do You Draw S P D F Orbitals Socratic
S Orbital Versus P Orbital While orbital numbers (eg, n = 1, 2, 3) indicate the energy level of an electron, the letters (s, p, d, f) describe the orbital shape The s orbital is a sphere around the atomic nucleus Within the sphere there are shells in which an electron is more likely to be found at any given time The smallest sphere is 1sAre to arrange an orbital of that shape around the nucleus "s" subshell One possible orientation "p" subshell Three possible orientations There are five possible orbitals in a "d" subshell, and 7 possible orbitals in an "f" subshell!Orbital Shapes (s, p, d and f) Explanation The proposed tetrahedral nucleus structure , along with rules for proton spin alignment that is the cause of the repelling force used to calculate orbital distances , can explain the shapes of the s, p, d and f orbitals


Shapes Of Orbitals And Sublevels


Vixra Org Pdf 1308 0130v1 Pdf
F orbitals are very complex and difficult to describe with words So there is only one kind of f orbitals and that is the f orbital I suppose you mean the differentFred Senese of Antoine Frostburg explains "You might expect that the 's' stands for 'spherical' and 'p' stands for 'polar' because these imply the shapes of the s and p orbitals, but unfortunately, the letter designations have nothing to do withAnswer to Describe the shapes of s p and d orbitals How arc these orbitals related to the quantum numbers n, t, and ml?


Q Tbn And9gcrp Takqfo4xfvuu Rp0n6n10v8innnzqycs3mwmp8itahq7xj Usqp Cau


Parsing The Spdf Electron Orbital Model
For a d orbital, draw a fourleafed clover;Protons forming in nucleus The f orbital has 15 protons to complete a fifth level of a tetrahedral structure Shape The f orbital is more complex, but follows the same rules based on proton alignment as the p and d orbitals When completely full it is similar to the d orbital, but cut in half (eight lobes instead of four)Atomic orbitals s, p, d, and f The s orbital is spherical in shape;


Chemistry Orbitals Chart The Future



Dublin Schools Lesson Orbital Diagrams And Electron Configurations
This video explains s, p, d, and f orbitals, sublevels, and their shapes It discusses the 4 quantum numbers n, l, ml, and ms n represents the energy leveThe subshells have four types s, p, d, and f, and each subshell has a specific number of orbitals with different shapes This is determined by the magnetic quantum number This is determined byHow Orbitals are oriented in space?shapes of s, p, d and f orbitals Orbitals In spaceHi!
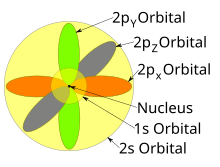


Atomic Orbital Wikipedia


Ch 9
The shapes of p, d and forbitals are described verbally here and shown graphically in the Orbitals table below The three porbitals for n = 2 have the form of two ellipsoids with a point of tangency at the nucleus (the twolobed shape is sometimes referred to as a "dumbbell"—there are two lobes pointing in opposite directions from each other)How Orbitals are oriented in space?shapes of s, p, d and f orbitals Orbitals In spaceHi!The shapes of p, d and forbitals are described verbally here and shown graphically in the Orbitals table below The three porbitals for n = 2 have the form of two ellipsoids with a point of tangency at the nucleus (the twolobed shape is sometimes referred to as a " dumbbell "—there are two lobes pointing in opposite directions from each other)


Q Tbn And9gcth1rc3hbnde1titk095wzz5fdzyo5obndscg8azgis25 Lq4re Usqp Cau
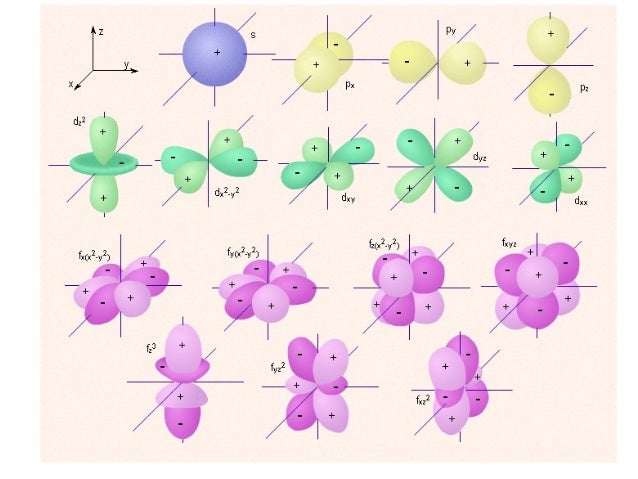


Orbital Shape Orientationt
Here you will learn all about your basic ideas, techniques, termiQuestion Question 3 A) Sketch The Shapes Of The S, P And D Orbitals State How Many Electrons Each Individual Orbital Can Contain As Well The Total Number In A Full S, P And D Subshell 10 Marks B) From The Following Section Of The Periodic Table He Be B с F Ne Sono 3 1 AI Si CI Show How The P Electrons Are Arranged In Be, B, C, N, O, F, Ne And State TheLarger elements have additional orbitals, making up the third electron shell Subshells d and f have more complex shapes and contain five and seven orbitals, respectively Principal shell 3n has s, p, and d subshells and can hold 18 electrons Principal shell 4n has s, p, d, and f orbitals and can hold 32 electrons


Atomic Orbitals



Mention The Shapes Of S P D F Orbitals Brainly In
Shapes Of S P And D Orbitals Pdf Download >>> DOWNLOAD (Mirror #1) d9ef92e1f7 embarkation card japan pdf downloadlouis claude fillion pdf downloadthe soul's code pdf downloadtoeic 4n4 860 level pdf downloadthe columbian exchange and global trade pdf downloadmcconnell economics 19th edition pdf free downloadghostgirl christmas spirit pdf downloadmanierismo y barroco pdf downloadcefaleaThe nucleus resides at the center of the sphere It does not orient itself in any direction In other words, it is nondirectional There are three dumbbellshaped p orbitals Each orbital has two lobes aligned in one of the three axesD and f orbitals In addition to s and p orbitals, there are two other sets of orbitals that become available for electrons to inhabit at higher energy levels At the third level, there is a set of five d orbitals (with complicated shapes and names) as well as the 3s and 3p orbitals (3p x, 3p y, 3p z) At the third level there are nine total



Orbitals Chemistry For Non Majors


S P D F Orbitals Chemistry Socratic
For an s orbital, draw a circle;
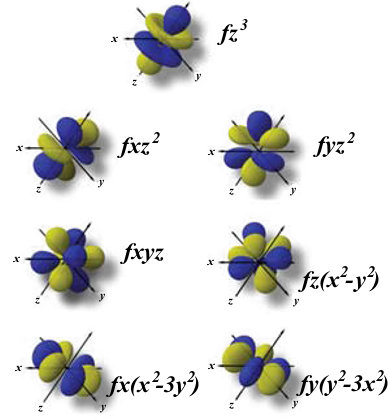


Atomic Orbitals Definition Shapes Examples And Diagrams


Shapes Of Orbitals And Sublevels
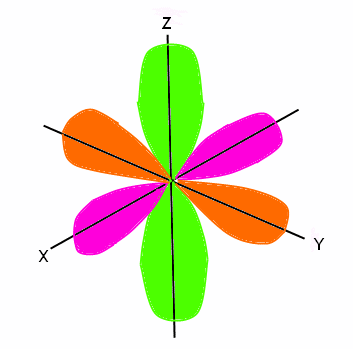


Electron Configuration Wyzant Resources


Q Tbn And9gcrfwbotnhjuu6xnuacit4rqntwaaundqvc5iwlz2bfb221zjkn Usqp Cau



Part 5 Shapes Of The Atomic Orbital S P D And F Orbital Atomic Structure Youtube



Quia Chem 4 2 The Quantum Model Of The Atom Bingo Version


Shape Of Orbitals Spdf Download Online Games



How To Draw All 5 D Orbitals Quora


Types Of Orbitals Mr Banks Fav Student
:max_bytes(150000):strip_icc()/energylevels-56a129545f9b58b7d0bc9f39-5aeb7f1aae9ab800373981a3.png)


S P D F Orbitals And Angular Momentum Quantum Numbers


Parsing The Spdf Electron Orbital Model



S P D F Obitals Notation Shapes Diagrams How To Work Out Electron Arrangements Configurations Order Of Filling Quantum Levels Electronic Structure Of Atoms Gce A Level Revision Notes



What Is The Structure Of An F Orbital Quora


Physics Revision Gcse And A Level Physics Revision Cyberphysics The Revision Website


Parsing The Spdf Electron Orbital Model


Home


2 2 Atomic Orbitals And Quantum Numbers Chemistry Libretexts


Parsing The Spdf Electron Orbital Model


Introduction To Electron Configurations Video Khan Academy



Powerpoint Orbital Shape Orientation Spdf Periodic Table Powerpoint Presentation Free Online Download Ppt 6tz333



Orbitals Chemistry Shapes Of Atomic Orbitals Shape Of S P D And F Orbital


Vixra Org Pdf 1308 0130v1 Pdf


Quantum Model And Spdf Orbitals Youtube



97 Atomic Orbitals W Adele Musicant Curioscity A Science Show


Orbitals A Level Chemistry


Orbitals Chemistry Shapes Of Atomic Orbitals Shape Of S P D And F Orbital



Chemistry 103 Lecture Ppt Video Online Download


S P D F Orbitals Chemistry Socratic


Atomic Structure Chemistry Encyclopedia Elements Metal Gas Number Name Symbol Equation Salt



Chemistry Online Shape Of Orbitals
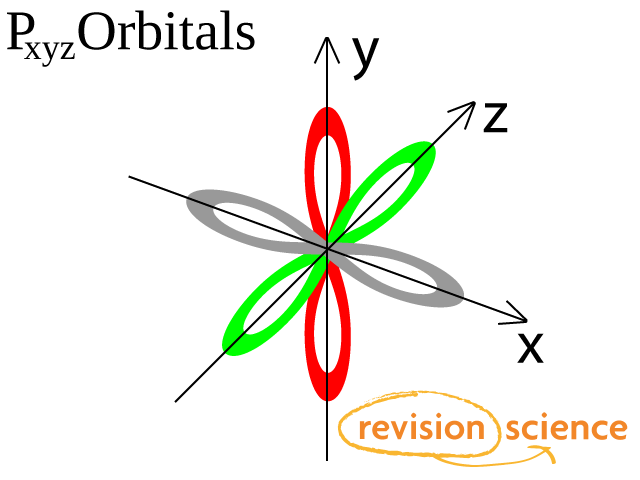


Orbitals A Level Chemistry



Draw The Shapes Of S P And D Orbitals Brainly In


Gsjournal Net Science Journals Research papers Chemistry Download 5032


An Atomic Model Our Present Model Of The Atom Is Based On The Concept Of Energy Levels For Electrons Within An Atom And On The Mathematical Interpretation Of Detailed Atomic Spectra The Requirements For Our Model Are Each Electron In A Particular Atom



Orbital



Introduction To Electron Configurations Video Khan Academy



Parsing The Spdf Electron Orbital Model Chemistry Education Chemistry Classroom Teaching Chemistry


Shapes Of Orbitals And Sublevels
:max_bytes(150000):strip_icc()/aufbauexample-56a129555f9b58b7d0bc9f48.jpg)


S P D F Orbitals And Angular Momentum Quantum Numbers



Orbitals Chemistry For Non Majors


Oneclass Com Homework Help Chemistry 163 How Do You Draw S P D F Orbi En Html



Orbitals The Basics Atomic Orbital Tutorial Probability Shapes Energy Crash Chemistry Academy Youtube



S P D F Orbitals



High School Chemistry Shapes Of Atomic Orbitals Wikibooks Open Books For An Open World
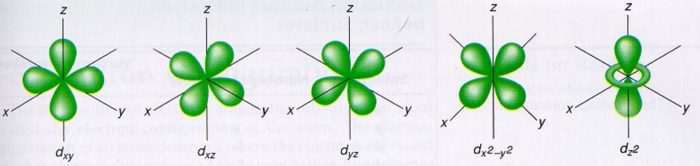


Shapes Of Atomic Orbital Chemistry Class 11 Structure Of Atom


Www Philadelphia Edu Jo Academics Ajaber Uploads Ch 6 Quantum theory and the electronicstructure of atom Part3 Pdf
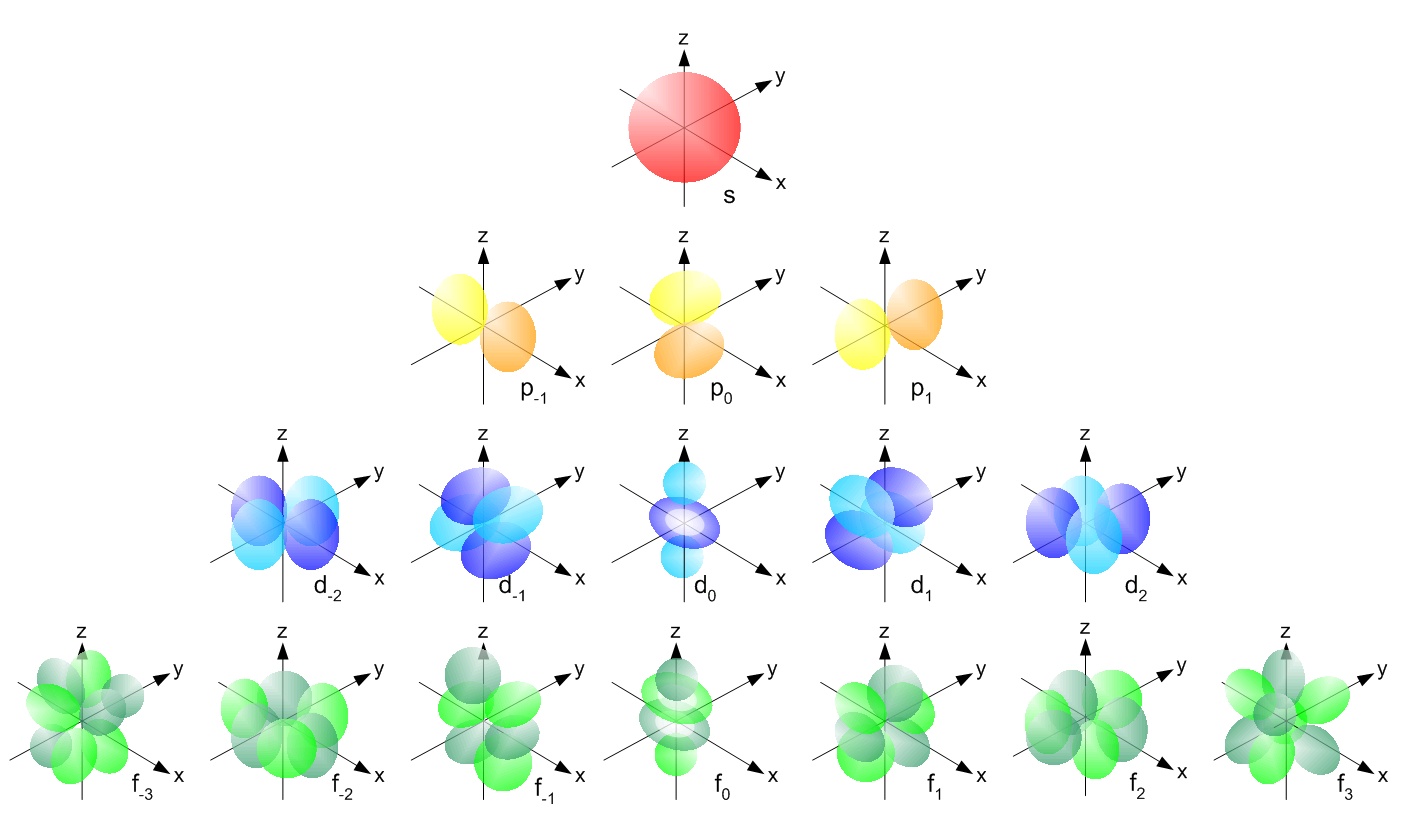


What Are The Orbital Shapes Of S P D And F Socratic
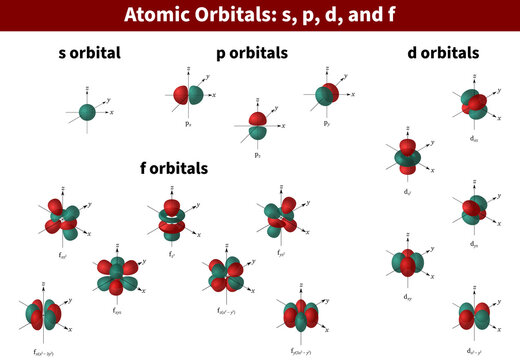


D Orbital Photos Royalty Free Images Graphics Vectors Videos Adobe Stock


S P D F Orbitals Chemistry Socratic



Shapes Of Atomic Orbitals Definition Examples Diagrams


Vixra Org Pdf 1308 0130v1 Pdf
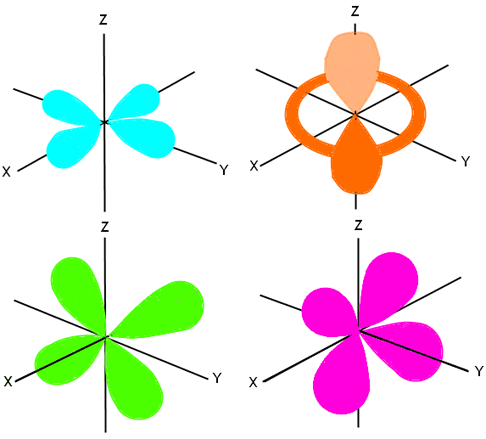


Electron Configuration Wyzant Resources


Parsing The Spdf Electron Orbital Model


Q Tbn And9gcqqfx6pzmsxdi2uw9vzetjpauxulquptk5uk642sgjdu4qcc5vw Usqp Cau



Quantum Mechanics Ppt Download



Shape Of Orbitals



Why Different Orbitals Have Different Shapes Britannica
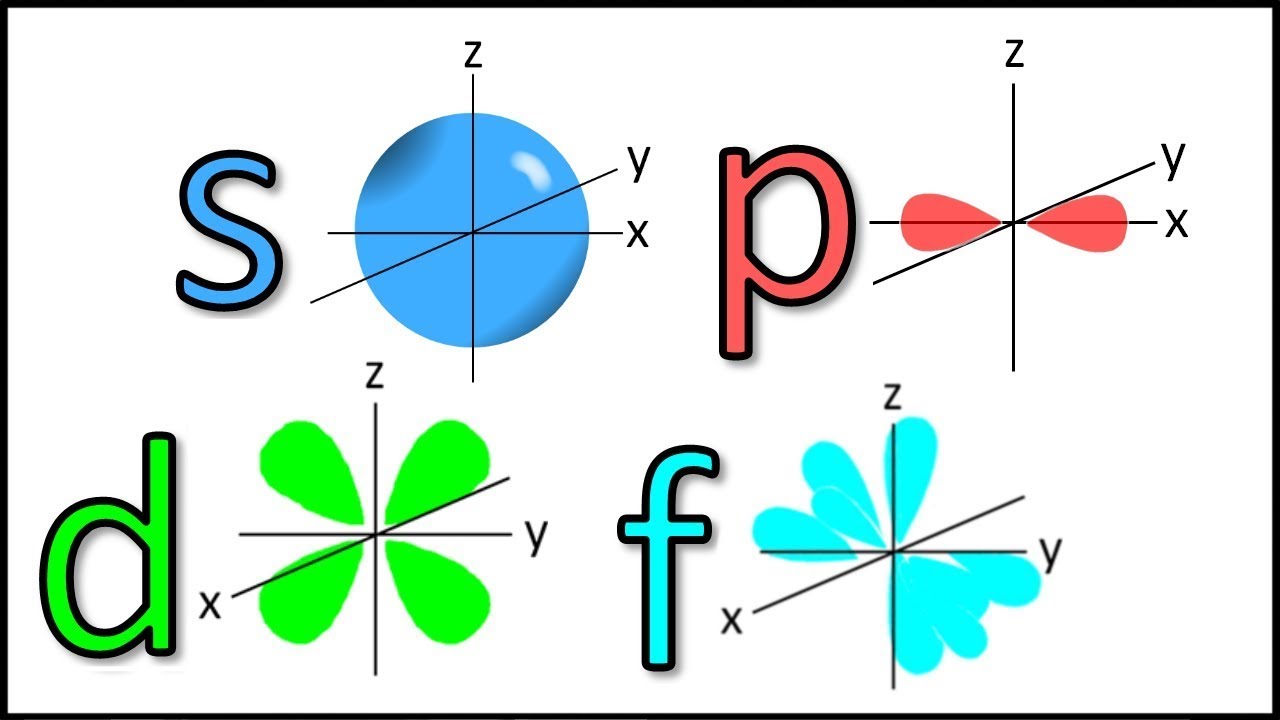


Atomic Orbitals And Its Shape Atomic Structure 11th Jee Neet Board In Hindi Youtube



Atomic Orbital Wikipedia


S P D F Orbitals Chemistry Socratic



Atomic Orbital Wikipedia



Shapes Of Orbitals Shape Of S Orbital P Orbital D Orbital F Orbital Node Angular Node Youtube



Orbital Shape Orientationt



What Is The Shape Of F Orbital Example
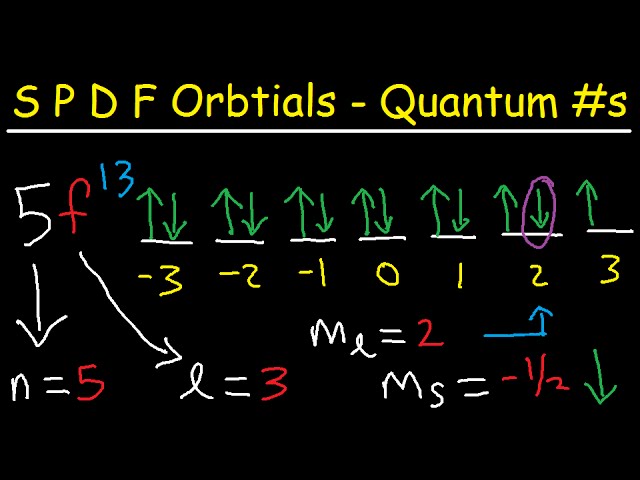


S P D F Orbitals Explained 4 Quantum Numbers Electron Configuration Orbital Diagrams Youtube



Shapes Of Atomic Orbitals Shape Determination Spd Videos Examples



What Is The Shape Of An F Orbital Quora
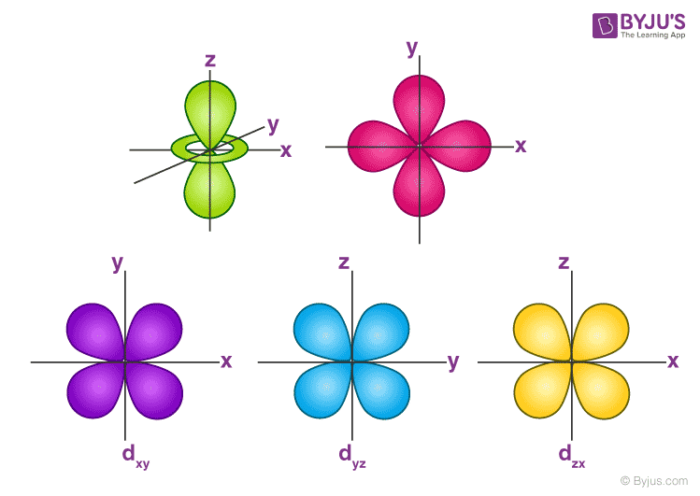


Orbitals Chemistry Shapes Of Atomic Orbitals Shape Of S P D And F Orbital



What Are Orbitals And Its Shapes S P D F Orbitals Shapes Class 9 Class 11 Chemistry Youtube Youtube


The Shape Of Orbitals Quantum Numbers


コメント
コメントを投稿